Over the past few years, blow out holes have appeared in the Arctic. They are often many tens of meters diameter and many tens of meters deep. They are close to round in shape with more or less vertical sides.
 | |
| Typical blow out hole. The people give scale. |
To my way of thinking, many of the commentators have got their wires crossed regarding how these features are formed. Let me run this by you.
1/ Much of the Arctic has permafrost, some of it multi-kilometers deep but much of it much less thick. By definition, permafrost is soil that is frozen over the summer and on into the next winter. Some of it has been frozen for thousands of years. Now with the warming of the climate, it is melting, especially at its more southerly extent. Leave that aside for a moment.
2/ When you mix methane with water, nothing happens. A very small amount of the methane dissolves in the water and the rest bubbles out. However cool the water to a few degrees above freezing and pressurize it to a pressure equivalent to about 250m deep in the sea or lake, and the methane combines with the water and forms an ice. It is called a methane ice, methane hydrate or Methane Clathrate*. A kg of water which is fully saturated with methane (forming an ice) can hold about 160liters of methane (measured at Standard Temperature and Pressure (STP)
*You can think of a Clathrate as a solid solution.
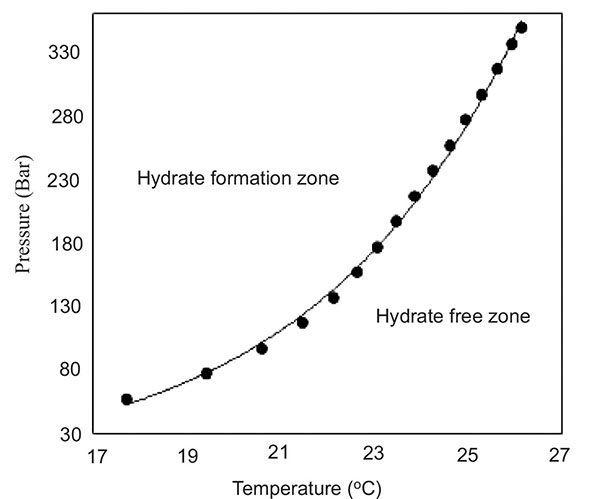
In fact under greater and greater pressure the methane clathrate can form at a higher and higher temperature. Note that to a first approximation, one bar is equal to one atmosphere. As you dive down in the ocean, again to a first approximation, for every 10 meters you descend, the pressure increases by one atmosphere (or one Bar). If you look at the above graph, you can see that a clathrate will form at about 180C at about 50 atmospheres(490m). This graph doesn't go far enough to the left but at a degree or two above freezing, a clathrate will form at about 25 atmospheres (Bar) or at about 250m*. Leave that aside for a moment.
*Methane ice has been dredged from the bottom of the ocean. A piece left on the deck slowly melts into a puddle of water. You can light it on fire and it burns as it gives up its methane.
| A piece of methane Clathrate burning. |
3/ When organic material is buried and especially if it finds itself at considerable depth where the heat of the earth is high*, the material breaks down into a range of hydrocarbons. It is basically a pyrolysis process. Depending on the starting material you can produce shale, coal or liquid hydrocarbons (oil) but in all cases, methane is one of the products. Being a gas the methane will seep upwards until it either meets an impermeable layer or it vents into the atmosphere. The impermeable layer can be a geological layer such as a layer of clay, for instance, or can be permafrost.
*The temperature in the earth is created by the decay of radioactive atoms and this heat slowly conducts and convects upwards to escape into the atmosphere. It is a minuscule amount compared to the heat we receive from the sun but since the earth is a great insulator, it accumulates and melts the center of the earth. As a rough rule of thumb, temperature increases about 250C per km depth.
4/ If the methane vents into the atmosphere, it comes into contact with OH (hydroxide) radicals and is slowly oxidized into Carbon dioxide and is incorporated into the biosphere. The half life of methane in the atmosphere is around 7 years. If it comes into contact with an impermeable layer, it accumulates. If there is moisture where it accumulates, if the pressure is sufficient, it forms a clathrate. A clathrate can form at much less than 250m depth if the impermeable layer confines the gas, like a pressure cooker lid. So let's put all this together.
Say you have a really solid, really cold impermeable layer of permafrost of 100m depth and somewhere below the permafrost there are layers of coal, oil or shale (or even a buried swamp or peat bog). They are venting methane but there is no geological impermeable layer between the hydrocarbon layers and the bottom of the permafrost. The bottom of the permafrost is at zero degrees C. If it was colder the permafrost would thicken (and there is moisture there). The permafrost acts like the lid of a pressure cooker and the methane begins to form a clathrate with the available moisture.
Now we humans come along and start to warm the atmosphere. The permafrost begins to warm and weaken and the clathrate warms as well. Gas comes out of the 'solid solution'. Pressure builds up. As mentioned a kg of clathrate can hold as much as 160liters of methane. If the pressure becomes high enough it blows a hole through the permafrost.
There is a corollary to this. Where we have continental glaciers, we should expect that there will be places where the glacier is underlying with coal, oil or shale. In such locations there should be a layer of methane clathrate at the bottom of the ordinary ice. The ice of a continental glacier can be a few km deep so the pressure is great and there are lakes under the ice of Antarctica. They remain liquid because of the heat seeping up from the earth and the insullating ability of the overlying ice. As the ice thins, the pressure decreases and so you would expect huge outputs of methane as the glaciers of Greenland and Antarctica retreat.
Incidentally, I have heard some commentators say that the ignition of the methane is what blows out these holes. Completely impossible. Methane only burns or explodes when mixed with air or oxygen at certain ratios. For methane this is between 5 and 60% methane. More than 60% methane and the mixture will not burn. Below the permafrost there is no oxygen, it is pure methane (probably with some Carbon dioxide). Once it enters the atmosphere, it could ignite but would need a source of ignition to burn or explode*.
*Owners of older style ICE (internal combustion engine) cars will be familiar with this. If you flood the engine the car will not start. You depress the accelerator, hold it there and try again. What you are doing is flushing out the excess fuel vapor. At too high a concentration it will not ignite.
