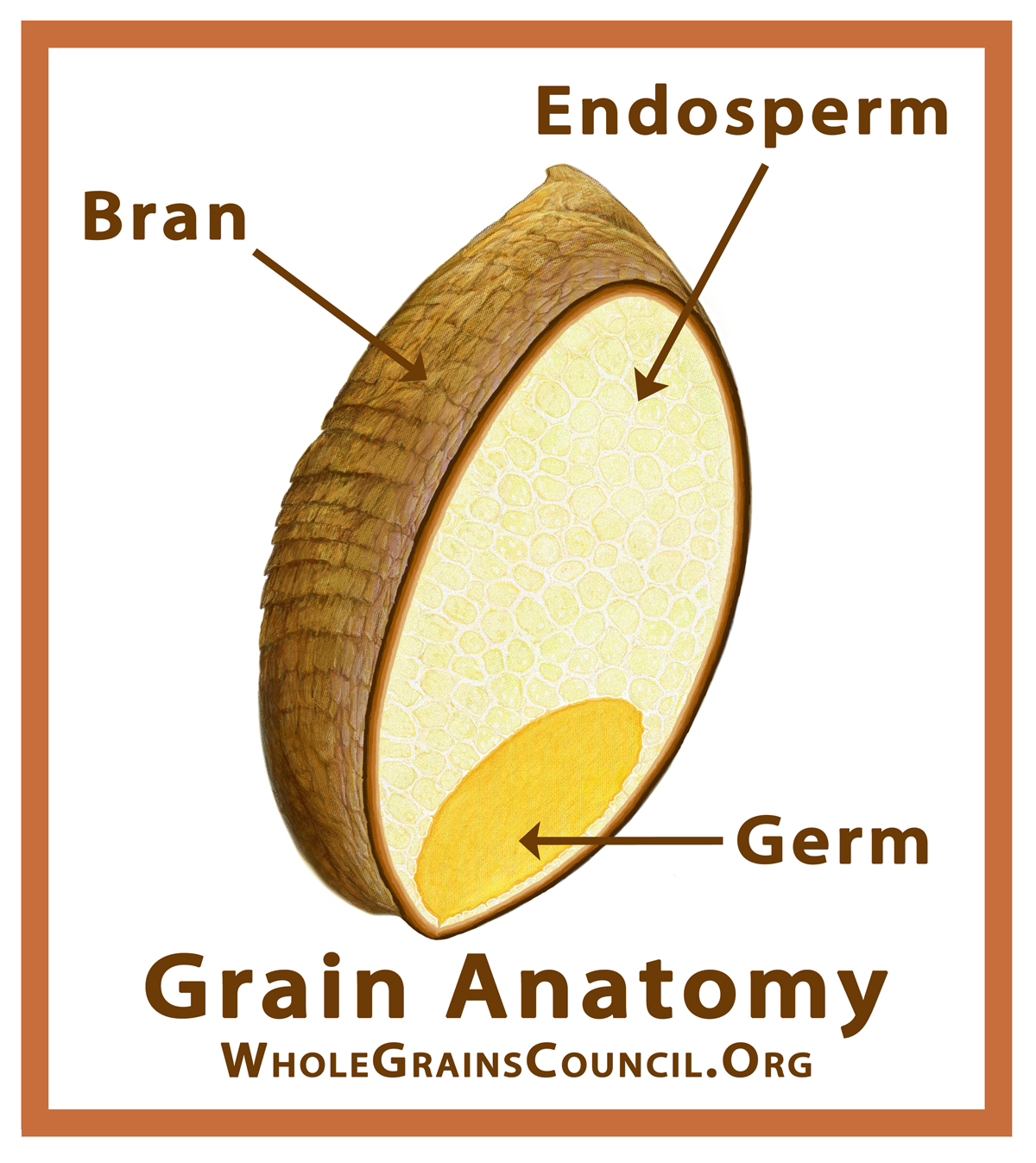
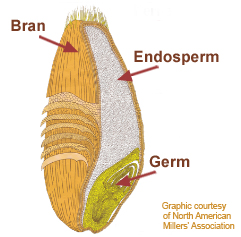
Possible effects of Latent Heat with regard to the melting of Greenland are interesting. As usual, this is speculation but based on old established physics. So what is Latent Heat.
When you add heat to an object it gets warmer. We will use the old imperial measurement since in this instance it is easier to understand.
A calorie (with a small 'c') was defined as the amount of heat needed to raise one gram of water by one degree centigrade. This is not Latent Heat. The term used is Sensible Heat - possibly because we can sense when something gets warmer. And, of course, it will take 100 calories to raise one gram of water, from zero degrees to the boiling point.
There are two types of latent heat. Lets start with the phase change from ice at zero degrees to water at zero degrees. For this transformation, it takes 80 calories to melt one gram. That is to say, the amount of heat to melt a gram of ice at zero degrees to water at zero degrees is the same as is needed to raise a gram of water from 00to 80 degrees Centigrade. This is the latent heat of the phase change between ice and water.
Importantly, when water becomes ice, exactly this amount of heat is given out. You might be tempted to say - "but won't this heat up the water". No. But it will keep the temperature at zero degrees centigrade until the water is all frozen. When ice is melting (say in a styrofoam cup) it will remain at zero degrees until all the ice is melted at which time the added heat from the environment will cause the water to warm.
The second latent heat is the phase change from water to water gas (water vapor). To convert a gram of water to water vapor takes 540 calories. This is 6.75 times as great as the phase change between ice and water. This will be important below.
Let's see what the importance may be of latent heat with respect to the great big ice cube which is Greenland or the even larger ice cube, Antarctica.
At some time in the not too distant future, all the ice will be gone on the Arctic ocean. Initially it will only occur in mid September when the ice minimum occurs but the period of no-ice will widen in subsequent years. Without ice, the heat absorbed by the open water will go into warming the water*. Here is our first effect of Latent heat, in this case the Ice-Water Latent heat. The ice will keep the water cold until it is all gone. When the ice is gone, the water begins to warm up.
* Actually this is a bit of an exaggeration. If you draw a cross section of the Arctic ocean to scale, it is a very shallow body of water in comparison to it's width. Already, for a considerable portion of the melt season, large areas are ice free. These are warming already since the ice that could keep them cool is far away across the ocean, but you get the idea.
As more and more of the water is ice free, we have ever warmer water on the surface of the Arctic ocean, heating the air from below and evaporating water vapor into the air. Since the solar radiation penetrates into the water, the warming occurs over one or two tens of meters of the surface, depending on the clarity of the water. It takes a lot of heat to warm water so the temperature only gradually increases but a very large amount of heat is stored in this surface water. It heats and humidifies the air blowing across the ocean*. What happens when this air blows across Greenland.
When you add heat to an object it gets warmer. We will use the old imperial measurement since in this instance it is easier to understand.
A calorie (with a small 'c') was defined as the amount of heat needed to raise one gram of water by one degree centigrade. This is not Latent Heat. The term used is Sensible Heat - possibly because we can sense when something gets warmer. And, of course, it will take 100 calories to raise one gram of water, from zero degrees to the boiling point.
There are two types of latent heat. Lets start with the phase change from ice at zero degrees to water at zero degrees. For this transformation, it takes 80 calories to melt one gram. That is to say, the amount of heat to melt a gram of ice at zero degrees to water at zero degrees is the same as is needed to raise a gram of water from 00to 80 degrees Centigrade. This is the latent heat of the phase change between ice and water.
Importantly, when water becomes ice, exactly this amount of heat is given out. You might be tempted to say - "but won't this heat up the water". No. But it will keep the temperature at zero degrees centigrade until the water is all frozen. When ice is melting (say in a styrofoam cup) it will remain at zero degrees until all the ice is melted at which time the added heat from the environment will cause the water to warm.
The second latent heat is the phase change from water to water gas (water vapor). To convert a gram of water to water vapor takes 540 calories. This is 6.75 times as great as the phase change between ice and water. This will be important below.
Let's see what the importance may be of latent heat with respect to the great big ice cube which is Greenland or the even larger ice cube, Antarctica.
At some time in the not too distant future, all the ice will be gone on the Arctic ocean. Initially it will only occur in mid September when the ice minimum occurs but the period of no-ice will widen in subsequent years. Without ice, the heat absorbed by the open water will go into warming the water*. Here is our first effect of Latent heat, in this case the Ice-Water Latent heat. The ice will keep the water cold until it is all gone. When the ice is gone, the water begins to warm up.
* Actually this is a bit of an exaggeration. If you draw a cross section of the Arctic ocean to scale, it is a very shallow body of water in comparison to it's width. Already, for a considerable portion of the melt season, large areas are ice free. These are warming already since the ice that could keep them cool is far away across the ocean, but you get the idea.
As more and more of the water is ice free, we have ever warmer water on the surface of the Arctic ocean, heating the air from below and evaporating water vapor into the air. Since the solar radiation penetrates into the water, the warming occurs over one or two tens of meters of the surface, depending on the clarity of the water. It takes a lot of heat to warm water so the temperature only gradually increases but a very large amount of heat is stored in this surface water. It heats and humidifies the air blowing across the ocean*. What happens when this air blows across Greenland.
*Incidentally, contrary to what 'the man in the street' might think, water vapor is only 60% as heavy as the same volume of dry air (obviously at the same temperature and pressure), so moist air is lighter than dry air. Note that water vapor does not disappear into the spaces between air molecules the way salt or sugar does when dissolved into water. Any gas adds its volume to the gas it is introduced into. Wet air over the Arctic ocean will tend to rise to be replaced by dry air from the surrounding continents (which will in turn take up moisture from the ocean and tend to rise)
First we must define The Lapse Rate. The Lapse Rate is the change in temperature if you take a body of air and increase it's altitude without the addition or removal of heat. For reasons, I won't go into, as air expands, it cools. Conversely as it is compressed, it warms. You can feel the practical effect of this if you pump up your tire with one of those cylindrical hand operated air pumps that you hold near the flexible tube that connects with the tire and pump with the other hand. The hand holding the tube gets hot.
Lapse rate is 9.8 degrees per km of altitude. That is to say, if I took a perfectly insulated balloon full of air and raised it up a kilometer, it would be 9.80C cooler at the top than when I started up.

It gets a tad more complicated when there is water vapor in the air (as there always is) but we will leave that for now.
Now, for the sake of the argument let's assume that we have fully saturated air at 100C blowing onshore in Greenland. The air hits the ice. Look at the following table. That 10 to the minus 3 kg/m cubed in the third column is their way of saying grams so a cubic meter of saturated air at 100c contains 9.39 grams of water in the form of water vapor.
| Temperature | Max. Water Content | ||
|---|---|---|---|
| (oC) | (oF) | (10-3 kg/m3) | (10-3 lb/ft3) |
| -25 | -13 | 0.64 | 0.040 |
| -20 | -4 | 1.05 | 0.066 |
| -15 | 5 | 1.58 | 0.099 |
| -10 | 14 | 2.31 | 0.14 |
| -5 | 23 | 3.37 | 0.21 |
| 0 | 32 | 4.89 | 0.31 |
| 5 | 41 | 6.82 | 0.43 |
| 10 | 50 | 9.39 | 0.59 |
| 15 | 59 | 12.8 | 0.8 |
| 20 | 68 | 17.3 | 1.07 |
| 30 | 86 | 30.4 | 1.9 |
| 40 | 104 | 51.1 | 3.2 |
| 50 | 122 | 83.0 | 5.2 |
| 60 | 140 | 130 | 8.1 |
This saturated air contacts the ice at 00C and the ice cools the air and causes water to condense out of the air. Remember that as water vapor changes into water, it gives out 540 calories per gram of water. Each gram of water condensed from the air gives out enough heat to melt six and three quarter grams of ice*.
*Incidentally if you want to read a dramatic account of a warm wind blowing across ice, read the book Plains of Passage by Jean Auel. True it is a novel but Jean did her homework and reports what generations of glaciologist have observed. It is somewhere around chapter 42 or 44. I can't find my copy of the book.
Let's back up a step. Where did this heat actually come from. The wind blowing across the open water is picking up the water vapor from above the ocean. Each gram of water that evaporates from the ocean takes this 540 calories from the ocean. So the air is cooling the ocean and the heat is being contained in the air as latent heat. If you have a wind that is blowing for some time from the water to the ice, a considerable amount of heat can be transferred. This is a convective process and convective processes are very powerful.
You remember, I said that the top ten or twenty meters of water are heated by the sun. As the surface water is cooled by the wind, it sinks and warm water comes to the surface. If the water has been open for a good portion of the summer, there is a lot of heat available.
Note that sun shining on snow isn't very good at melting it. Most of the radiation is reflected back to space without warming the snow. Clear ice or ice with a pool of water on its surface is a little different. The radiation penetrates but has to heat a considerable layer of ice up to zero degrees C before melting starts.
A warm wind or a wind with lots of water vapor is something else again. The heat is applied on the very surface of the ice and is constantly replenished from the sea. If there is considerable water vapor in the wind, latent heat of condensing water vapor is added to the sensible heat of the wind.
If this was dry wind blowing across Greenland, it would only contribute sensible heat to the ice. The air would cool both by contact with the ice and the expansion of air as it rose up the slope. However with a high water vapor content, some of the latent heat of the condensing water vapor stays in the air.
You remember, in our example we started with 10 degrees C, fully saturated air. At a little over a km in altitude, it would have cooled to zero degrees and would stop melting the ice. However some of the latent heat which is released as water vapor condenses into droplets (fog), the air will remain above zero degrees to a higher altitude, all the while melting the ice.
Of course the situation get's rapidly worse as the air becomes warmer than the 100 C we took as our example and the water vapor content of the air increases. Have a look back at the table.
While we are at it, there is another scenario that may be relevant to the story of a melting Greenland.
Suppose there isn't much wind but Greenland is bathed in war moist air right to the top. This air is light (relatively) due both to it's temperature and it's water vapor content. That's right. Humid air is lighter than dry air. The reason is interesting and explained below. It is in contact with the ice. The ice cools this air and condenses out some of the water vapor making it heavier. If the droplets of water stay in the air as fog, this exacerbates the effect. This air now begins to flow down the slope as a density current.
You remember the lapse rate. It works in the other direction too. For every km that this air flows down the slope (vertical kilometer), it warms by 9.8 degrees C. by compression. Of course, it doesn't actually warm. It transfers this heat to the ice, melting it. These are the the famous Piteraqs that are seen around the shores of Greenland.
A body of air flowing from the very top of Greenland to the sea would warm almost 30 degrees if it didn't gain or loose heat. This heat plus the latent heat of water vapor condensing on the ice is available to melt the ice. We should see some rather extreme melting events in the future.
Relative density of gases
Gases have some interesting properties. The volume of a gas is inversely related to pressure (if you keep temperature constant). That is to say, if you double the pressure, you half the volume. The volume of a gas is directly related to temperature. Not Centigrade but Kelvin temperature otherwise known as absolute temperature. This is temperature measured from absolute zero. If you increase the temperature of a liter of a gas, for instance, from zero degrees centigrade (2730K) to 100 degrees centigrade (3730K) then the volume will increase by 373/273 = 1.37liters.
Leaving all this aside, let's get on to the really interesting aspect of gases. It turns out that a given volume of any gas at the same temperature and pressure contains the same number of particles. I say particles rather than atoms since many gases exist as molecules of two atoms such as N2, O2 and H2. This has an interesting implication. If you know what gas you have, you can work out it's relative density to, for instance, air.
Now air is a combination of mainly Nitrogen and Oxygen. An atom of Nitrogen has an atomic weight of 14 so each N2 atom is 28. Oxygen, similarly has an molecular weight of 32. So air is approximately 30 (I should have done a weighted average but we are just illustrating the principle). Water vapor consists of two hydrogen atoms and one oxygen atom so has a relative weight of 18. Water vapor is only 18/30 = 3/5ths or 60% as dense as air. Now we need one more property of gases.
When you put sugar into water it dissolves and to some extent the sugar fits between the water molecules. The volume of the sugar and the water is somewhat less than the volume of the water and the sugar added together. Gases are not like this. Each molecule occupies the same volume as any other molecule. So if you add a tenth of a liter of water vapor to a liter of air (with no condensation, of course) you end up with 1.1 liter of gas.
You can see, therefore, that humid air which is a mix of water vapor (relative density 18) and air (relative density 30) is lighter than dry air.
All bets are off, of course, if the water vapor condenses into fog. Now you have a suspension of water droplets in air and it is heavier than dry air.
So we have a couple of mechanisms that could cause a rather striking acceleration in the melting of the surface of Greenland.