There is a tipping point in both the Arctic and Antarctic that is not much talked about that would result in the rather sudden disappearance of sea ice with various unpleasant results.
For instance if there was no sea ice formed over the respective winters, then as soon as the sun returned, the water would begin to absorb all the impinging solar radiation. At present, even in the worse case, at the beginning of the 'light-season', there is a lot of sea ice reflecting light back into space and solar radiation is progressively absorbed, throughout the spring and summer, as the ice cover decreases.
In the Antarctic, land-glaciers would flow more quickly into the ocean, increasing the rate of sea level rise. At present, sea ice acts like a 'cork in the bottle', slowing down the flow of land glaciers.
In the arctic, with the increased heat in the water, Walker cells* could well establish themselves between the ocean and the ice of Greenland, accelerating the melting of her ice sheet. Snow reflects solar radiation back into space. Clear ice must be heated to a considerable depth before melting occurs. But warm winds are a different situation all together. If they persist for any length of time they carve away at the surface ice
*Air rising over the ocean, flowing toward Greenland where the ice cools it, extracts its heat and moisture making it denser** and it flows along the ice, back down to the ocean. As it flows down in a density current, it heats by compression giving it even more ice melting capacity.
**Note that contrary to expectations, dry air is heavier than humid air.
The tipping point I am talking about depends on a bit of well established physics and while I don't say it is guaranteed to happen, there are indications that it is already heading that way at both poles.
First, the behavior of sea water and fresh water as it cools. In both cases the density increases but with a couple of differences. First, water with salt dissolved in it is denser than fresh water and the more salt, the greater the density. No surprises there. Second as water cools it contracts and gets denser (heavier per unit volume if you like). But there is one difference between fresh and salt water. Salt water gets more and more dense right down to it's freezing point and its freezing point is a degree or two below 00 C depending on how salty it is. Fresh water on the other hand gets denser and denser down to 40 C and then gets less dense as the water molecules begin to link up. It freezes at 0 degrees Centigrade.
All this results in a curious effect. In a deep body of sea water (the ocean) sea ice can not form unless you have a halocline (a layer of fresher water floating on the deeper denser full salinity sea water. Note I didn't say fresh water but rather fresher water.
For instance, a halocline is formed in a sea where melting land ice has added a lot of fresh water to the ocean, which mixes with the sea water, and creates a layer of less salty water which floats on the deeper water. Or can occur in an ocean in which rivers from the surrounding land are pouring fresh water into the ocean. So why is a halocline needed for the production of sea ice.
In a deep ocean, with no halocline, as the surface layer cools, it becomes denser and sinks down into the depths. Warmer salty water comes up to replace it and the cycle continues. You would need a far longer cold period than the ~half year 'dark period' during the winter in the Arctic or Antarctic to cool this whole depth of water to the point where ice could begin to form. Once it started to form, the situation would change dramatically since as sea water freezes, it expels much of its salt which then dissolves in the remaining sea water making it really dense. This denser water would then plunge into the depths. But this could only occur if the world got really cold for a long period, so that the whole depth of the ocean was severely cooled. This may be what happened during the period known as snow ball Earth, but I digress.
When there is a sufficient halocline, the cold air above the ocean only has to cool the upper layer. It is still sea water, albeit fresher than deeper water and the convection cycle with denser water sinking, only occurs in this limited layer of fresher water. Even at its greatest density, just before the freezing point, the surface water is not dense enough to sink through the deeper, more salty water. An important point here is that both layers don't have to be exactly at the same salinity for freezing to be stopped. It only requires that the density is different enough that the surface water at its freezing point isn't denser than the deeper water. If for any reason, the salinity of the surface water increases sufficiently, the creation of sea ice would stop. I'll leave the maths boffins to work out at what combinations of salinity of the surface and deep water that this would occur.
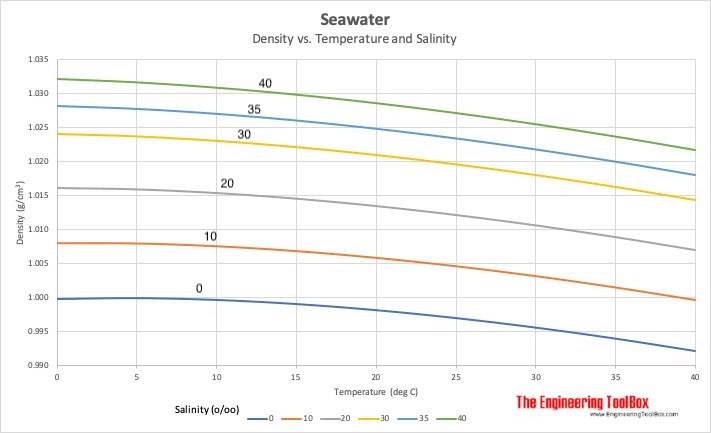
While the above graph gives the over all situation, unfortunately for the lowest line - the one showing fresh water, it is incorrect. It should show the density of fresh water decreasing below 4 degrees centigrade.
Note that open ocean water is generally about 35 ppm.
Let's do a wee thought experiment using the above graph. Assume that the underlying deep ocean water is at 35 (probably pretty close to the true situation) and the upper, fresher layer was at 30. Could it ever sink into the lower level. Looking at the graph, the maximum density of salt water containing 30kg of salt per ton of sea water is about 1.024. Going across the chart at this level, we don't hit the 35kg/ton curve until we get to about 220C. Since the deep water is somewhere under 50C, 30 water could never sink through 35 water. It looks as if the salinity of the upper layers would have to get very close to the salinity of the lower layers before mixing, caused by the cooling of the surface layer, could occur.

Here is a better graph of the cooling of fresh water. Note when it actually freezes , the density of ice is 0.917 grams per cc. (the density of ice at 0 degrees C.). As ice cools below 0 C, it contracts a little.
So, what we are looking for is any mechanism that would increase the salinity of the surface layers of sea water enough to stop ice formation in the winter. Remember if this happens, and there is open water when the 'light season' begins then solar radiation would be absorbed by the ocean for the whole of the light period and not just as the ice melts, exposing some open water.
Note also, that the intensity of radiation per square meter of ocean surface, is not as intense as it is per square meter directly under the sun. The radiation over the area around the poles comes in at a slant, and so the same amount of solar radiation is spread over a greater area. However, the sun shines for longer and longer, during the 'light period'. Close to the poles, the light shines 24 hours a day.
The second bit of physics we need is the Coriolis effect. On earth everything that moves in the Northern Hemisphere is veered to the right by Coriolis. You can look up many excellent items on YouTube, which explain why this is. Everything moving in the Southern Hemisphere is veered to the left. The effect, for obvious reasons, (Obvious, once you have looked up why Coriolis occurs) gets greater and greater the closer you are to one of the poles.
Let's start with the Antarctic.
It has been observed over the past couple of years that the salinity of the surface water has been increasing. This was something of a surprise since, with the warming of the Antarctic, more ice is melting and flowing out on to the ocean. This is simply an observed fact and if it is the beginning of a trend, we could get to the point where sea ice can not form in the winter. Here are a couple of speculations, suggesting why the surface water is becoming more salty.
If you look down on the Antarctic from space, the winds go clockwise around the coast. This pushes on the water and makes it flow in the same direction. Remember if something is at rest, Coriolis has no effect on it but when it moves it is veered to the left and the faster it moves, the greater the effect. In a clockwise rotating body of water in the Southern Hemisphere, to the left is away from the coast. Perhaps the fresher surface layer is being pushed more strongly toward the North, bringing up more salty deeper water. It has been reported that these winds have been getting stronger.
Another possibility is that storms are getting stronger with bigger waves that mix the surface layers more deeply. If more deep water is mixed with the surface water, its salinity will increase*.
* One additional effect is that if any of these effects increases the depth of the halocline, there is more water to be cooled before sea ice formation can start. Remember, you have to get this surface water down to the freezing point before ice will begin to form.
And one more possibility. The sea ice has shown a dramatic decrease over the past few years after a long period in which it was slowly increasing. When there is a good cover of sea ice, it suppresses the effect of winds on the underlying ocean. As the sea ice decreases, even the same amount of wind has an increased effect of mixing surface layers. A very negative, positive feed-back mechanism. Less ice and more wind is doubly effective in mixing surface layers with deeper water.
Whatever the cause, observations over the past couple of years show an increase in surface salinity.
So how about the Arctic.
In the 'classic' Arctic, sea ice completely covers the ocean even well into summer. The ice reflects most of the light impinging on it. In the rest of the world, solar radiation goes right through the atmosphere to be absorbed by the land. The heated land heats the air in contact with it, causing it to become less dense and rise. In the Arctic, when there is complete ice and snow cover, there is very little heating from below. The air radiates heat into space* and cools, becoming more dense. The air falls, spreading out to the South as it hits the land. Coriolis veers this land-surface wind to the right resulting in the Polar Easterlies. The air flows to about 60 degrees North, heating as it goes and rises and returns North at altitude. This circulation is called the Polar Hadley cell.
*Anything that is above 00K radiates heat. If heat is not coming in from somewhere it cools.
So you have the classic high pressure, clockwise rotating system in the Arctic. In a clockwise rotating system in the Northern Hemisphere, to the right is into the center. Look at the Beaufort gyre in the Arctic. It retains floating ice and the fresher layer of water and in fact is slightly higher in the middle than surrounding water. I think that you see where this is going.
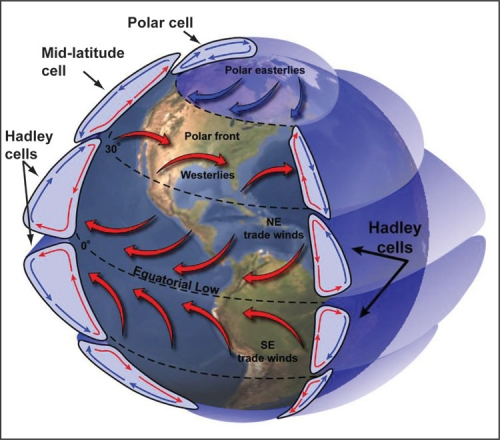
If we melt enough ice in the Arctic with our output of Green House Gasses and particulates, the Arctic as it absorbs more solar radiation could flip to rising air rather than falling air. This might be most likely to first be observed in the fall when the land is cooling quickly while the sea is still heating the air above it from its greater accumulation of heat. This would be a classic low pressure counter clockwise rotation. In a counter clockwise rotational system, to-the-right is away from the center. In other words, If the Beaufort gyre reverses direction, it would be expelling ice and floating water away from the center to be expelled through the Bearing and Fram Straits.
As in the Antarctic, thinner or no ice allows the winds to mix the layers more easily, and more deeply, increasing the salinity of the surface layers and deepening the halocline which is now saltier than previously. As in the Antarctic, a deeper halocline has to be cooled to the freezing point of water of its salinity before sea ice formation can start.
A summer of deep drought one year in the catchment that provides fresh water to the Arctic ocean, could also decrease the salinity of the ocean's surface layers.
One other effect, out of left field, which could occur is an underwater earth quake which causes a Tsunami to race back and forth across the Arctic Ocean. Waves don't occur just on the interface between air and water. They also occur between layers of different salinity and hence density. And they break as they reach shallower water, just as air-water waves do. This might mix the layer sufficiently to flip us into a new mode in which the halocline is sufficiently disrupted to stop the formation of sea ice.
A man made effect is also possible. The Halocline in the Arctic is created, largely by the influx of fresh water from North American and Russian rivers. If this water was diverted to the south for agriculture, this could also weaken the halocline.
So in conclusion, if the surface layers get sufficiently salty in both the Arctic and Antarctic to stop the formation of sea ice in the winter, then all the sun energy for the whole light period would be absorbed, making it unlikely that sea ice would ever form again and providing much more heat to melt both Antarctica and Greenland glaciers. Talk about tipping points!!
